
VIVA QUESTIONS
Q1. How are cements classified based on their function?
Ans. Classification of Cements:
-
Liners
-
Bases
-
Restorative materials
-
Luting agents
Q2. What are pulp protecting agents?
Ans. ‘Pulp protecting agents’ are materials used to protect pulp from chemical, electrical, thermal, or mechanical injuries. They are cavity varnishes, liners, and bases that are used as adjuncts to the restorative materials.
Q3. What are cavity varnishes?
Ans. ‘Cavity varnishes’ are solutions of natural or synthetic resins in volatile liquids, like chloroform, alcohol, or acetone.
Q4. Where and how is a cavity varnish applied?
Ans. Cavity varnish is applied on all the prepared surfaces of the cavity, walls and floor, including margins of the cavity. It is applied in 2−3 coats with a cotton pledget.
Q5. What is the film thickness of cavity varnish?
Ans. 5−10 μm thickness.
Q6. What are the uses of cavity varnish?
Ans. It seals the dentinal tubules and thus protects the pulp and prevents postoperative sensitivity.
Q7. Where is a varnish contraindicated?
Ans. It cannot be given under a glass ionomer restoration, as it hinders adhesion of the material to the tooth structure. It retards polymerization of composite resins and blocks resin tags and hence
cannot be used with composite restorations.
Q8. What is a base?
Ans. ‘Base’ is a cement placed below a restoration to protect the pulp against thermal injury, galvanic shock and chemical irritation, and provide mechanical support for the restoration.
Q9. What are the functions of a base?
Ans: The functions of a base are:
-
It provides thermal insulation.
-
It prevents chemicals from the restorations leaching into the pulp through dentinal tubules.
-
It prevents bacterial penetration into the pulp through marginal leakage.
-
It provides mechanical support for the restoration and distributes local stresses to dentin.
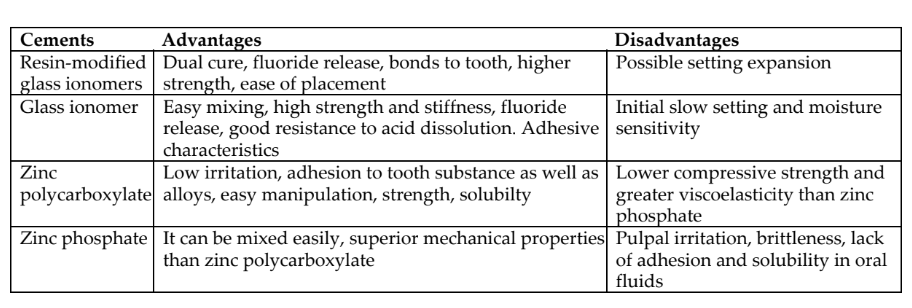
Q10. What are the materials of choice based on the remaining dentin
thickness (RDT)?
Ans: Cavity with remaining dentin greater than about 0.5 mm can be restored with resin-modified glass ionomer as the first material of choices followed by glass ionomers, zinc polycarboxylate, and zinc phosphate. Cavity with minimal dentin or pulpal exposure calcium hydroxide salicylate or MTA (mineral trioxide aggregate) are the material of choices.
Q11. What is the thickness of a base?
Ans: Minimal thickness of 0.75 mm is recommended.
Q12. What is cavity liner?
Ans: Cement given in the deepest portion of a cavity for a specific therapeutic effect, e.g. Ca (OH)2 and MTA.
Q13. What is MTA?
Ans: A tri- and dicalcium silicate-based cement used for vital pulp therapy and endodontics indications including apexification, iatrogenic perforation repair, resorption repair, root-end filling, and sealing.
Q14. What is the composition of MTA?
Ans: Composition of MTA
-
Tricalcium silicate 45−75 wt%
-
Dicalcium silicate 7−32 wt%
-
Bismuth oxide or tantalum oxide 20−35 wt%
-
Tricalcium aluminate 0−13 wt%
-
Gypsum 2−10 wt%
Q15. Should base be applied on the gingival seat?
Ans: No, it should not be applied on the gingival seat.
Q16. Where do we apply the base?
Ans: Base is applied on the pulpal floor and axial wall.
Q17. Why should the base not be applied on the gingival seat?
Ans: When base is applied on the gingival seat, it might get dissolved or it will be disintegrated by the action of saliva, as it is open to the external margin. It leads to microleakage, which may further lead to
secondary caries.
Q18.What is the composition of zinc phosphate cement?
Ans: It is available as powder and liquid.
-
Powder consists of zinc oxide 90.2%, magnesium oxide, saliva, and other oxides.
-
Liquid consists of phosphoric acid and water.
Q19. How should zinc phosphate cement be mixed?
Ans: It should be mixed over a broad area preferably on a cool glass slab to dissipate exothermic heat. Powder is divided into smaller increments and each increment is incorporated in the liquid and mixed.
Q20. What is the setting time of zinc phosphate cement?
Ans: 2.5−8 min.
Q21. What is the pH of zinc phosphate cement?
Ans: Approximately 2 at the start of mixing, then increases and reaches 5.5pH at 24 H.
Q22. What is the powder to liquid ratio for zinc polycarboxylate
cement?
Ans: 1.5:1
Q23. Why is zinc polycarboxylate cement preferred over zinc phosphate clinically?
Ans: Because:
-
Zinc polycarboxylate cement has good biocompatibility compared to zinc phosphate cement, as its pH rises rapidly during the setting reaction and also due to the larger size of polyacrylic acid, which limits its diffusion into the dentinal tubules
-
It also bonds to the tooth structure chemically.
-
Zinc phosphate cement causes pulpal irritation due to its low pH and due to the ability of the smaller-sized phosphoric acid molecules to diffuse into the dentinal tubules.
Q24. How is zinc phosphate mixed?
Ans: Powder is divided into smaller increments and each increment is incorporated into the liquid and mixed.
Q25. What is the pH of calcium hydroxide cement?
Ans: 11.5−12 pH
Q26. What is the action of calcium hydroxide as cavity liners ? why is calcium hydroxide the preferred choice as cavity liners?
Ans: Calcium hydroxide is used as a sub-base due to its pulpal biocompatibility, antibacterial activity, and ability to stimulate reparative dentin formation.
Q27. What is the mechanism of reparative dentin formation by calcium hydroxide?
Ans: Calcium hydroxide induces mild necrosis of pulpal tissue, which stimulates the odontoblasts which in turn forms reparative dentin bridge.
Q28. What is the mechanism for the antibacterial effect of calcium hydroxide?
Ans: High pH of Ca(OH2) is not conducive for survival of most bacteria, and hydroxyl ions formed by ionic dissociation have lethal effect on cytoplasmic membrane of bacteria.
Q29. What is microleakage?
Ans: ‘Microleakage’ is defined as the passage or ingress of bacteria and their toxins between the restoration margins and tooth preparation walls.
